Clinical Trials
of Tampa
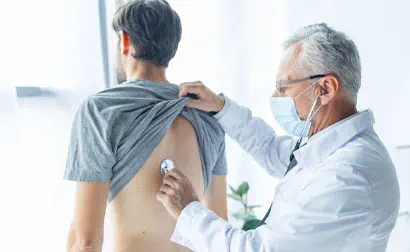
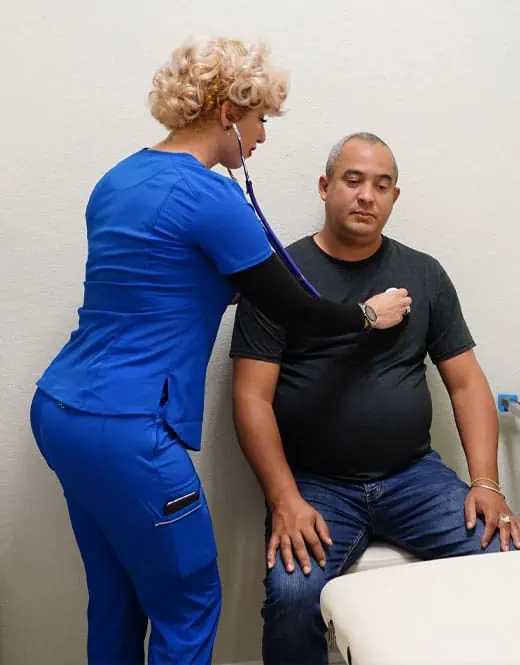
When people like you volunteer to participate in clinical trials, we can uncover better ways to treat conditions that affect thousands of people. You can help make a difference.
Clinical Trials of Tampa
About Our Volunteer Focused Clinical Trials
Our mission is to help develop specialized drugs and therapies through clinical research, and we couldn't do it without you, the volunteer. We have had many volunteers just like you help us impact the world by participating in a clinical trial. We are making a difference by:
Contributing to knowledge about new treatments.
Offering access to new investigational treatments.
Providing information about the patient's disease.
Giving regular and careful medical care.
Years of Combined Experience
WHAT WE OFFER
Current Trials Studies in Tampa

Participate
Why should I volunteer?
Taking part in a clinical trial is a deeply personal decision for you and your family. Many volunteers take part to find potential new treatments for a specific condition while others do it to help advance modern medicine for others in the future. Many people volunteer to take advantage of the benefits. Whatever your reason, your health and safety are always our top priority.
You may be one of the first to receive a new type of medication that is not yet available to the public.
All medical exams, tests, and procedures related to the trial will be provided to you at no cost.
You may receive additional care from healthcare providers
You may also receive financial compensation for your time and travel
10+
Studies conducted
300+
Patients enrolled
2-3
Completed phases
2+
Therapy areas
The clinical trial journey
The clinical research process

Select a clinical trial


apply


Be accepted


Informed consent

Get screened


Medication administration


Payment/compensation

Follow-up
Active Studies
Alzheimer
Asthma
Colonoscopy
FAQ
General Questions
If you choose to participate in a Phase I clinical trial, it's important to know that you likely won't experience any therapeutic benefits from the medication being tested. Instead, you'll be compensated for your participation, which usually includes reimbursement for time and any inconvenience experienced during the trial. Payments are typically made after the trial is completed, and how they're made will be described in the Informed Consent Form that you'll receive before beginning the trial.
Each trial is different, so your involvement will vary based on the trial’s pre-determined plan, or protocol. For some, you may need to stay in a study clinic for a few nights. For other trials, you may simply need to visit a study clinic every couple of weeks. Before you begin, all time requirements will be explained to you.
A research team will likely include many people behind the scenes. You may interact with doctors, nurses, healthcare professionals, data managers, and clinical trial coordinators at different times during your participation.